Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2191
Peer-review started: July 13, 2014
First decision: August 15, 2014
Revised: October 27, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: February 21, 2015
AIM: To investigate semaphorin 4D (Sema4D) and hypoxia-inducible factor-1α (HIF-1α) expression in colorectal carcinoma and evaluate their clinicopathological and prognostic significance.
METHODS: Eighty-six curatively resected colorectal carcinoma patients at different stages of disease were randomly selected from the group of patients who underwent surgery, and none of them received preoperative radiochemotherapy. Normal proximal adjacent bowel tissue, which served as an internal control, was obtained from 52 randomly selected patients. Immunohistochemistry was performed to analyze the expression of Sema4D and the tumor angiogenesis-related protein HIF-1α in normal colorectal tissues and colorectal carcinoma tissues. The relationships between the expression and clinical characters and prognosis were analyzed.
RESULTS: HIF-1α and Sema4D were positively expressed in 58% and 60% of colorectal carcinoma tissues, respectively. Significantly lower expression levels were observed in normal mucosa (8% and 12%, respectively). HIF-1α and Sema4D expression was closely correlated with histological tumor type, tumor-node-metastasis (TNM) stage, and lymphatic metastasis (P < 0.05), but not with age or tumor size (P > 0.05). HIF-1α and Sema4D protein expression was significantly correlated with prognosis of colorectal carcinoma, as determined by Spearman rank correlation analysis (r = 0.567; P < 0.01). Multivariate Cox analysis revealed that only Sema4D expression played a significant role in predicting patient prognosis (P < 0.05).
CONCLUSION: These findings suggest that HIF-1α and Sema4D expression correlates with histological tumor type, TNM stage, and lymphatic metastasis in colorectal carcinoma and that Sema4D is a prognostic indicator of colorectal carcinoma.
Core tip: Although originally identified in the context of neurodevelopment, semaphorin 4D (Sema4D) is also important in angiogenesis. Sema4D is widely expressed in several types of malignant solid tumors. However, its function and expression in colorectal carcinoma are not understood. This study indicates that Sema4D expression is positively correlated with HIF-1α in colorectal carcinoma and that Sema4D is a novel indicator of poor prognosis for colorectal carcinoma patients. We expect that Sema4D could be used as a reliable tool to the early and accurate prediction of tumor recurrence, and it represents a potential therapeutic target for colorectal cancer patients.
- Citation: Wang JS, Jing CQ, Shan KS, Chen YZ, Guo XB, Cao ZX, Mu LJ, Peng LP, Zhou ML, Li LP. Semaphorin 4D and hypoxia-inducible factor-1α overexpression is related to prognosis in colorectal carcinoma. World J Gastroenterol 2015; 21(7): 2191-2198
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2191.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2191
Colorectal cancer ranks among the most common malignancies, and it is the fourth leading cause of cancer-related death throughout the world[1,2]. The United States has a yearly report of over 148000 new cases of colorectal cancer[3], and China is facing an increase in the incidence of this carcinoma. Despite extensive research efforts, many patients ultimately die from disease recurrence. Thus, to improve the diagnosis and prognosis of colorectal cancer, it is of great importance to get a full understanding of the molecular mechanisms underlying its carcinogenesis.
Tumor hypoxia is a key factor that leads to the development of malignancy because it promotes the release of factors essential for angiogenesis and tumor growth. Solid tumors generally grow in a faster pace than their local blood supply, which results in a hypoxic microenvironment. Though lesions 1-2 mm in diameter get nutrients through cellular diffusion, an increase in tumor size beyond this level needs fast adaptation to hypoxia so as to keep the growth and necrosis from being cessative[4]. The master regulatory protein involved in the cellular response to oxygen levels is the hypoxia-inducible factor (HIF). HIF-1 is a heterodimer consisting of two components: HIF-1α and HIF-1β. HIF-1α is oxygen-regulated and determines the activity of HIF-1. Under hypoxic conditions, the transcriptional activity of HIF-1 grows at a speedy pace for the over-expression of HIF-1α protein[5]. HIF-1 binds to hypoxia response elements (HREs) in the promoters of target genes and activates their transcription for adaptation to decreased oxygen concentrations (i.e., the formation of new blood vessels through the proliferation of endothelial cells as well as their migration toward the developing tumor)[6]. This response is influenced by the increased production of pro-angiogenic proteins, such as vascular endothelial growth factor (VEGF). In addition, semaphorins may be regulated by changes in oxygen tension, which is the same case with some other pro-angiogenic factors[7]. Semaphorins are a large family of membrane-bound or secreted proteins originally described in the nervous system[8,9]. These molecules play important roles in angiogenesis, for they regulate blood vessel growth and endothelial cell homing during vessel development[10]. A recent study indicated that Sema4D, originally discovered to regulate B cell aggregation and survival as well as T cell activation in the immune system[11,12], is induced by hypoxia in an HIF-1-dependent manner. Sema4D effects tumor vascularity in head and neck squamous cell carcinoma in such a way that is analogous to that of VEGF, attracting plexin-B1-expressing endothelial cells into the tumor to promote growth and vascularity[13].
Eighty-six curatively resected colorectal carcinoma patients at different stages of disease were randomly selected from the group of patients who underwent surgery between January 2004 and June 2005 at the Provincial Hospital Affiliated to Shandong University. None of the patients received preoperative radiochemotherapy. Among these patients, 44 were female and 42 were male, and their ages ranged from 32 to 73 years. According to a prospective protocol, the tumor and all of the lymph nodes were cut at several levels and embedded in paraffin, and sections were obtained for routine hematoxylin and eosin (HE) staining. Experienced pathologists examined the slides and documented the pathological characteristics of the tumor and lymph nodes. Tumor stage was defined according to the tumor-node-metastasis (TNM) staging system. The data obtained at regular follow-up visits to the outpatient department were stored in a database specifically designed for colorectal carcinoma patients. An update regarding the present status of all surviving patients was obtained by phone or mail in December 2012. Normal proximal adjacent bowel tissue, which served as an internal control, was obtained from 52 randomly selected patients.
Immunohistochemical staining for HIF-1α and Sema4D was performed on 4-μm-thick paraffin-embedded sections of tumor tissues using the streptavidin-biotin-peroxidase complex technique (ABC). After deparaffinizing and rinsing the slides several times with PBS, endogenous peroxidase activity was inactivated by incubating the slides in 3% H2O2 in methanol for 20 min at room temperature, and nonspecific binding was blocked by incubation in 5% bovine serum albumin for 20 min. Antigen retrieval was performed by microwaving the sections for 25min in either 10mmol/L sodium citrate (pH 6.0) or 0.05mol/L Tris-HCl (Sigma-Aldrich Ltd., Poole, United Kingdom)/1 mmol/L EDTA (Sigma) (pH 8.5 or 9.0) buffer solution. Sections in different groups were then incubated with a rabbit monoclonal anti-HIF-1α or anti-SEMA4D (Boster Biotechnology Company, BA1278, 1:50) for 1 h at 37 °C. Then, the slides were incubated with an HRP-conjugated secondary antibody (goat anti-mouse IG) at 37 °C for 30 min. The sections were then stained by the ABC method, using DAB as a substrate, for 10 min; HE staining was used as a counterstain. As a negative control, slides were processed with no primary antibodies. To account for batch-to-batch variation, two sections with high and low HIF-1α expression were identified, with more sections from these biopsies being included in each batch.
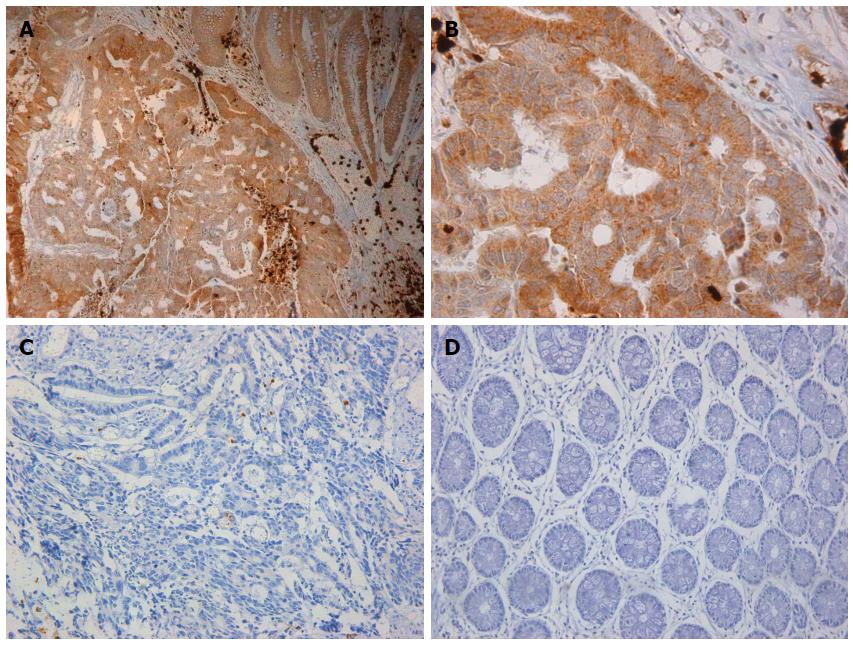
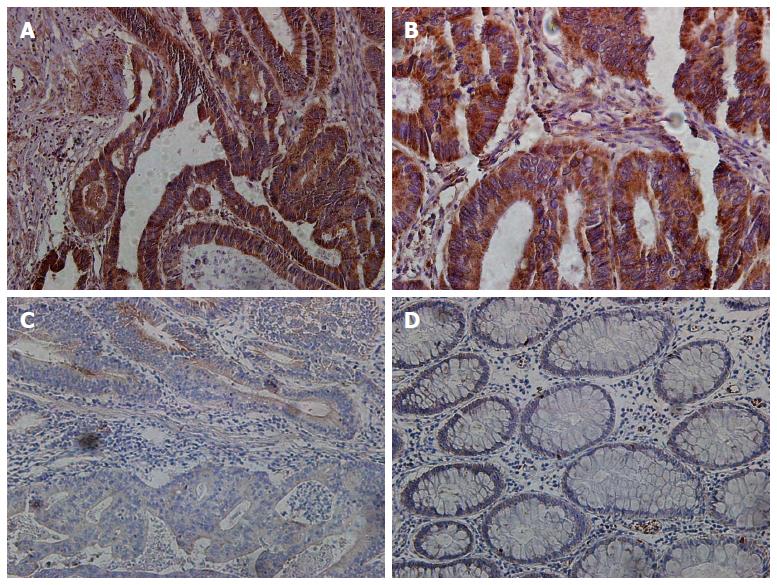
Scoring was performed in a double-blind manner by two investigators. All disagreements were resolved by discussion to obtain final scores. Brown staining in the membrane, cytoplasm, and nuclei of tumor cells was regarded as indicating positive expression of HIF-1α or Sema4D. Staining results were evaluated according to the percentage of cells with positive staining and staining intensity in the cytoplasm of cells. For scoring, five high-power fields (× 400) were selected in each section to count positive cells, and the sections were divided into four groups according to the proportion of positive cells as the following: (1) 0 points, percentage of positive cells ≤ 5%; (2) 1 point, percentage of positive cells > 5% and ≤ 25%; (3) 2 points, percentage of positive cells > 25% and ≤ 50%; (4) 3 points, percentage of positive cells > 50% and ≤ 75%; and (5) and 4 points, percentage of positive cells > 75%. The scores for staining intensity were defined in the cytoplasm of cells as follows: (1) 0 points, no coloring; (2) 1 point, light yellow; (3) 2 points, brown-yellow; and (4) 3 points, tan. Final scores were calculated as the score of positive cells multiplied by the score of staining intensity in the cytoplasm. According to this final score, the samples were divided into two grades: negative (0-2) and positive (3-7)[14].
The correlation between HIF-1α and Sema4D expression and a variety of clinicopathological parameters was determined by Student’s t-test, Fisher’s exact test, or the Mann-Whitney U test, as appropriate. Differences in the frequencies between different groups were determined using the χ2 test. The correlation between the two variables was determined using Spearman’s rank test. Survival curves were plotted using the Kaplan-Meier method, and statistical significance between groups was determined by the log-rank test. The end point for analysis was overall survival beginning from the surgery day. Independent variables predicting survival were estimated by multiple step-by-step regression analysis using the Cox model. All statistical tests were two-sided, with differences being reckoned significant at the 0.05 significance level. As for data analysis, SPSS13.0 software was used.
Figures 1 and 2 display photomicrographs of HIF-1α and Sema4D staining in normal mucosa and cancer tissues. Immunohistochemistry revealed that 58% (50/86) and 60% (52/86) of colorectal cancer tissues stained positive for HIF-1α and Sema4D, respectively, significantly higher than those in normal tissues (8% and 12%, respectively, P = 0.00). Both HIF-1α and Sema4D were occasionally expressed in the luminal border of the normal mucosa. However, this staining was weak and cytoplasmic, with no nuclear staining observed in normal mucosal cells. By way of contrast, the staining pattern was variable in tumors. HIF-1α expression was observed in the cytoplasm and/or nuclei of tumor cells, and the staining was prominent in the advancing border of the tumor. Interstitial cells, most likely macrophages, were frequently positive for both HIF-1α and Sema4D. Occasionally, cytoplasmic staining for Sema4D was observed as aggregates at the luminal border of tumor cells.
Among the 50 cancer tissues which stained positive for HIF-1α, 84.0% (42/50) stained positive for Sema4D. However, among the 36 cancer tissues that stained negative for HIF-1α, 27.8% (10/36) stained positive for Sema4D. Thus, we observed a significant correlation between Sema4D and HIF-1α expression (r = 0.567; P < 0.01; Table 1).
| n | Sema4D-negative | Sema4D-positive | |
| HIF-1α-negative | 36 | 26 (72.2) | 10 (27.8) |
| HIF-1α-positive | 50 | 8 (16.0) | 42 (84.0) |
Fifty-two (60%) and 50 (58%) tumors were respectively positive for Sema4D and HIF-1α. Table 2 displays the association between Sema4D and HIF-1α expression and the clinicopathological variables. Overexpression of Sema4D and HIF-1α was significantly connected to the advancement of lymphatic metastasis and the TNM stage of the tumor. A significant correlation can be observed between the differentiation status of the tumor and expression of Sema4D and HIF-1α, with increased Sema4D and HIF-1α expression found in moderately and poorly differentiated tumors.
| HIF-1α-positive | Sema4D-positive | ||||||
| n | n (%) | χ2 | P value | n (%) | χ2 | P value | |
| Age (yr) | 0.013 | 0.911 | 0.130 | 0.719 | |||
| ≤ 50 | 40 | 23 (58) | 25 (63) | ||||
| > 50 | 46 | 27 (59) | 27 (59) | ||||
| Differentiation | 8.5791 | 0.0031 | 6.6641 | 0.0101 | |||
| High | 28 | 10 (36) | 11 (39) | ||||
| Medium | 42 | 30 (71) | 28 (67) | ||||
| Low | 16 | 10 (63) | 13 (81) | ||||
| Tumor size | 0.225 | 0.636 | 0.624 | 0.429 | |||
| < 5 cm | 50 | 28 (56) | 32 (64) | ||||
| ≥ 5 cm | 36 | 22 (61) | 20(56) | ||||
| TNM Stage | 8.728 | 0.003 | 5.657 | 0.017 | |||
| I-II | 30 | 11 (37) | 13 (43) | ||||
| III-IV | 56 | 39 (70) | 39 (70) | ||||
| Lymphatic metastasis | 8.579 | 0.003 | 5.385 | 0.020 | |||
| Yes | 58 | 40 (69) | 38 (66) | ||||
| No | 28 | 10 (36) | 14 (50) | ||||
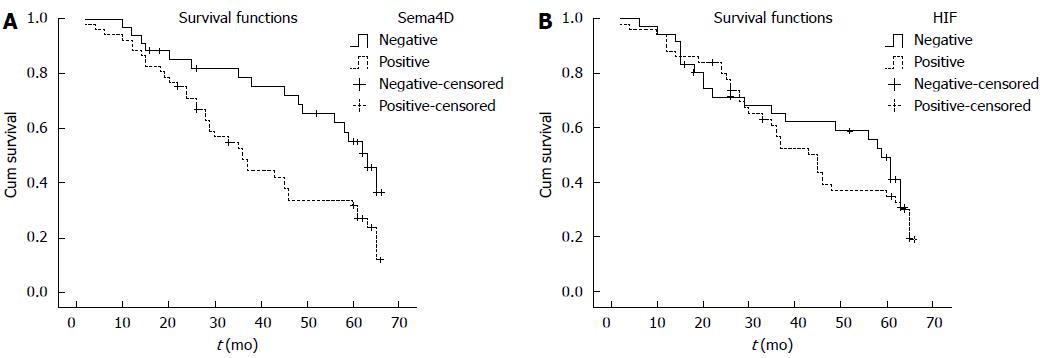
Univariate analyses revealed that patient prognosis was relevant to the differentiation status, TNM stage, lymphatic metastasis, as well as the expression of HIF-1α and Sema4D (P < 0.05; Table 3). Moreover, multivariate Cox analysis revealed that TNM stage and positive Sema4D expression had a significant, independent effect on patient prognosis, with HIF-1α expression being eliminated during the step-by-step multivariate analysis in contrast (Table 4). Overall speaking, patients with positive Sema4D expression survived a shorter time in comparison to those with negative expression (Figure 3).
| 5-yr survival rate | ||||
| n | n (%) | χ2 | P value | |
| Age (yr) | 1.664 | 0.197 | ||
| ≤ 50 | 40 | 12 (30) | ||
| > 50 | 46 | 20 (43) | ||
| Differentiation | 4.7571 | 0.0291 | ||
| High | 28 | 15 (54) | ||
| Medium | 42 | 13 (31) | ||
| Low | 16 | 4 (25) | ||
| Tumor size | 0.058 | 0.810 | ||
| < 5 cm | 50 | 18 (36) | ||
| ≥ 5 cm | 36 | 14 (39) | ||
| TNM stage | 5.127 | 0.024 | ||
| I-II | 30 | 16 | ||
| III-IV | 56 | 16 | ||
| Lymphatic metastasis | 7.061 | 0.008 | ||
| Yes | 58 | 16 (28) | ||
| No | 28 | 16 (57) | ||
| HIF-1α | 8.920 | 0.003 | ||
| Negative | 36 | 20 (56) | ||
| Positive | 50 | 12 (24) | ||
| Sema4D | 11.244 | 0.001 | ||
| Negative | 34 | 20 (59) | ||
| Positive | 52 | 12 (23) | ||
| Overall survival | |||
| HR | 95%CI | P value | |
| Differentiation | 0.653 | 0.373-1.144 | 0.136 |
| Tumor size | 1.315 | 0.859-2.012 | 0.208 |
| TNM stage | 0.920 | 0.529-1.601 | 0.768 |
| Lymphatic metastasis | 2.499 | 1.345-4.641 | 0.004 |
| HIF-1α | 1.802 | 0.9.0-3.489 | 0.081 |
| Sema4D | 0.780 | 0.402-1.513 | 0.462 |
| 2.147 | 1.157-3.983 | 0.015 | |
HIF-1 drives or represses the transcription of many genes and pathways that are critical for the coordination of oxygen supply and cellular metabolism. Rapidly growing tumors with high metabolic demands, genetic mutations, or other tumorigenic events, along with the influence of inflammatory mediators and other tumor and stromal-released growth factors, are unable to properly degrade HIF-1α, resulting in the stabilization and activation of the HIF-1 transcriptional complex[5]. Recent studies showed that HIF-1α expression is on an increase in many primary and metastatic tumors, which suggests that its stabilization is a common consequence of a wide range of mutations underlying human cancer[15]. The HIF-1 heterodimer binds to HREs in the cis-acting sequences of target genes, many of which are involved in adaptive pathways, such as angiogenesis[16]. Notably, previous studies demonstrated that the angiogenic response induced by Sema4D was comparable to that of other famous angiogenic molecules, including VEGF, HGF, and bFGF, and it depended on the up-regulation of these molecules[17-21].
Our study, which encompassed a large group of colorectal cancer cases across all disease stages, indicated that both HIF-1α and Sema4D are extensively expressed in cancer tissues in comparison to normal large bowel mucosa (controls). The tissues in more than half of all cases expressed HIF-1α (58%), and almost two-thirds of the tissues expressed Sema4D (60%). Overexpression of Sema4D and HIF-1α was closely related to the advancement of lymphatic metastasis and the TNM stage of the tumor, and there was a significant correlation between the expression of these proteins and the differentiation status of the tumor, with increased expression present in moderately and poorly differentiated tumors. However, multivariate Cox analyses revealed that only TNM stage and positive Sema4D expression had a significant, independent effect upon patient prognosis, and HIF-1α was eliminated from the step-by-step multivariate analysis. Sema4D expression had a significant effect upon patient prognosis in terms of overall survival. This study indicates that Sema4D was expressed in a subgroup of patients with aggressive colorectal carcinoma, and this protein is an independent prognostic marker in colorectal carcinoma patients. In comparison with the frequent expression of Sema4D in more advanced tumor stages, HIF-1α was expressed to a similar extent in early- and advanced-stage tumors.
Tumor-infiltrating macrophages induce angiogenesis in colorectal cancer by releasing thymidine phosphorylase[22]. Moreover, Leek et al[23] reported that HIF expression in macrophages strongly enhanced tumor angiogenesis in breast carcinoma. Recently, Sema4D expression, which is controlled by the HIF family of transcription factors, was found to collaborate with VEGF to promote tumor growth and vascularity in oral squamous cell carcinoma[24]. In addition, Sema4D collaborates with VEGF to promote angiogenesis in malignancies, and it can compensate for VEGF inhibition[25]. Therefore, our data suggest that HIFs differentially regulate the transcription of major angiogenic factors during the early and late phases of tumor angiogenesis. The lack of prognostic impact of HIF1 could be explained by the intricate relationship between HIF1 and the mutation of tumor suppressor genes. In ovarian carcinoma, HIF1 overexpression alone shows no prognostic value, and it only showed strong prognostic value when combined with nonfunctional p53 protein[26]. A similar correlation may also be discovered in colorectal carcinoma, and further studies are required to solve this problem. In the present study, macrophages stained strongly positive for Sema4D and HIF-1α. Additional studies are needed to address this issue.
In conclusion, this paper indicates that Sema4D expression is positively correlated with HIF-1α expression in colorectal carcinoma, and that Sema4D is a novel indicator of poor prognosis for colorectal carcinoma patients. Conversely, HIF-1α overexpression was observed in the early and late hypoxic phases of colorectal carcinoma. Thus, targeting specific angiogenic factors at different stages of colorectal tumor development could represent an effective treatment strategy. Sema4D may be applied as a reliable tool to the early and accurate prediction of tumor recurrence, and it represents a potential therapeutic target for colorectal cancer patients.
Hypoxia plays an important part in a large number of biological processes including tumorigenesis and angiogenesis, and hypoxia-inducible factor-1 alpha (HIF-1α) plays a key part in this process. As a new proangiogenic factor, Semaphorin 4D (Sema4D) is widely expressed in several malignant solid tumors, though originally identified in neurodevelopment. Nevertheless, the function and expression of Sema4D in colorectal carcinoma are as yet not well understood.
HIF-1 drives or represses the transcription of many genes and pathways that are critical for the coordination of oxygen supply and cellular metabolism. Rapidly growing tumors with high metabolic demands, genetic mutations, or other tumorigenic events, along with the influence of inflammatory mediators and other tumor and stromal-released growth factors, are unable to properly degrade HIF-1α, resulting in the stabilization and activation of the HIF-1 transcriptional complex. Recent studies showed that HIF-1α expression is on an increase in many primary and metastatic tumors, which suggests that its stabilization is a common consequence of a wide range of mutations underlying human cancer. The HIF-1 heterodimer binds to HREs in the cis-acting sequences of target genes, many of which are involved in adaptive pathways, such as angiogenesis. Notably, previous studies demonstrated that the angiogenic response induced by Sema4D was comparable to that of other famous angiogenic molecules, including VEGF, HGF, and bFGF, and it depended on the up-regulation of these molecules.
This study, which encompassed a large group of colorectal cancer cases across all disease stages, indicated that both HIF-1α and Sema4D are extensively expressed in cancer tissues in comparison to normal large bowel mucosa (controls). Overexpression of Sema4D and HIF-1α was closely related to the advancement of lymphatic metastasis and the tumor-node-metastasis (TNM) stage of the tumor, and there was a significant correlation between the expression of these proteins and the differentiation status of the tumor, with increased expression present in moderately and poorly differentiated tumors. However, multivariate Cox analyses revealed that only TNM stage and positive Sema4D expression had a significant, independent effect upon patient prognosis, and HIF-1α was eliminated from the step-by-step multivariate analysis. Sema4D expression had a significant effect upon patient prognosis in terms of overall survival. This study indicates that Sema4D was expressed in a subgroup of patients with aggressive colorectal carcinoma, and this protein is an independent prognostic marker in colorectal carcinoma patients. In comparison with the frequent expression of Sema4D in more advanced tumor stages, HIF-1α was expressed to a similar extent in early- and advanced-stage tumors.
This paper indicates that Sema4D expression is positively correlated with HIF-1α in colorectal carcinoma, and that Sema4D is a novel indicator of poor prognosis for colorectal carcinoma patients. Conversely, HIF-1α overexpression was observed in the early and late hypoxic phases of colorectal carcinoma. Thus, targeting specific angiogenic factors at different stages of colorectal tumor development could represent an effective treatment strategy. Sema4D may be applied as a reliable tool to the early and accurate prediction of tumor recurrence, and it represents a potential therapeutic target for colorectal cancer patients.
The authors investigated Sema4D expression in colorectal carcinoma and evaluated its clinicalpathological and prognostic significance. The results suggest that the expressions of HIF-1α and Sema4D are correlated with histological types, TNM stages and lymphatic metastasis in colorectal carcinoma, and Sema4D can be used as a prognostic indicator of colorectal carcinoma. The manuscript is very interesting, some minor revisions needed.
P- Reviewer: Koji N, Sugano S S- Editor: Yu J L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 3. | American Cancer Society. Cancer facts and figures. Atlanta, GA: American Cancer Society 2006; . [Cited in This Article: ] |
| 4. | Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1738] [Cited by in F6Publishing: 1698] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 5. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [PubMed] [Cited in This Article: ] |
| 6. | Vajkoczy P, Farhadi M, Gaumann A, Heidenreich R, Erber R, Wunder A, Tonn JC, Menger MD, Breier G. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest. 2002;109:777-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Siebold C, Berrow N, Walter TS, Harlos K, Owens RJ, Stuart DI, Terman JR, Kolodkin AL, Pasterkamp RJ, Jones EY. High-resolution structure of the catalytic region of MICAL (molecule interacting with CasL), a multidomain flavoenzyme-signaling molecule. Proc Natl Acad Sci USA. 2005;102:16836-16841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13:79-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 709] [Cited by in F6Publishing: 746] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 742] [Cited by in F6Publishing: 743] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 11. | Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA. 1996;93:11780-11785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 216] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, Yukawa K, Ikawa M, Okabe M, Parnes JR. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Sun Q, Zhou H, Binmadi NO, Basile JR. Hypoxia-inducible factor-1-mediated regulation of semaphorin 4D affects tumor growth and vascularity. J Biol Chem. 2009;284:32066-32074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693-4696. [PubMed] [Cited in This Article: ] |
| 15. | Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 594] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 16. | Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 614] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 17. | Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood. 2005;105:4321-4329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212-5224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Liu B, Ma Y, Jin B. Sema 4D/CD100-plexin B is a multifunctional counter-receptor. Cell Mol Immunol. 2013;10:97-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Chen Y, Zhang L, Lv R, Zhang WQ. Overexpression of Semaphorin4D indicates poor prognosis and prompts monocyte differentiation toward M2 macrophages in epithelial ovarian cancer. Asian Pac J Cancer Prev. 2013;14:5883-5890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Liu H, Yang Y, Xiao J, Yang S, Liu Y, Kang W, Li X, Zhang F. Semaphorin 4D expression is associated with a poor clinical outcome in cervical cancer patients. Microvasc Res. 2014;93:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Matsumura M, Chiba Y, Lu C, Amaya H, Shimomatsuya T, Horiuchi T, Muraoka R, Tanigawa N. Platelet-derived endothelial cell growth factor/thymidine phosphorylase expression correlated with tumor angiogenesis and macrophage infiltration in colorectal cancer. Cancer Lett. 1998;128:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res. 2002;62:1326-1329. [PubMed] [Cited in This Article: ] |
| 24. | Zhou H, Yang YH, Binmadi NO, Proia P, Basile JR. The hypoxia-inducible factor-responsive proteins semaphorin 4D and vascular endothelial growth factor promote tumor growth and angiogenesis in oral squamous cell carcinoma. Exp Cell Res. 2012;318:1685-1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Zhou H, Binmadi NO, Yang YH, Proia P, Basile JR. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis. 2012;15:391-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G. Expression of hypoxia-inducible factor 1alpha in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res. 2001;7:1661-1668. [PubMed] [Cited in This Article: ] |